Your Cart is Empty

YOUR CART
Your cart is empty!
Add your favorite items to your cart.

Zantac Recall And Alternatives
December 10, 2019 7 min read
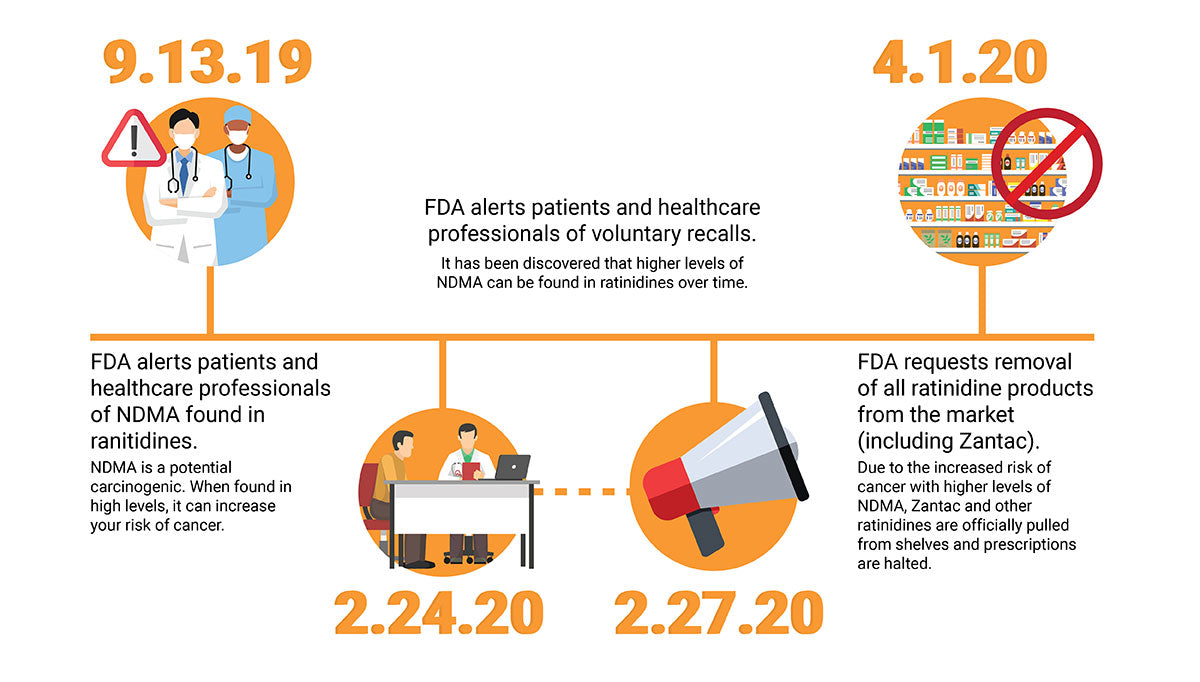
If you suffer from gastroesophageal reflux disease (GERD), ulcers, or stomach-acid-related conditions, you may rely on ranitidine products, most commonly Zantac, in order to find relief. However, according to FDA.gov, as of April 1, 2020, the FDA has officially pulled Zantac and other ranitidine drugs from the market.
The reason for the recall? It has been found that traces of a known carcinogen were found in some of the products, making the risk of danger too high for consumers. In compliance, many major pharmacy chains such as CVS Health Corp and Walgreens have already announced that they have pulled Zantac and generic versions of the drug from their shelves and will stop selling them immediately.
As such, this recall leaves millions of heartburn sufferers seeking safe and effective alternative treatments. However, that can be easier said than done if you’ve been taking Zantac or another ranitidine drug and aren’t sure what your other options are. After all, if it had been working, it’s unlikely that you looked into it further.
If you find yourself in this situation, wondering how you’ll get the relief you need, use this guide to learn more about your options. To learn more about what ranitidine drugs are, the Zantac recall, Zantac alternatives, and how you can better manage your condition, read from start to finish. Or, you can use the links below to go directly to a specific section.
- Why Was Zantac Recalled?
- Zantac Alternatives
- Next Steps: What Should You Do If You’ve Taken Zantac in the Past?
Why Was Zantac Recalled?
The FDA announced that small amounts of N-nitrosodimethylamine (NDMA) were found in samples of ranitidine, most commonly marketed as Zantac in the US and Canada. NDMA has been linked to an increased risk of colorectal and uterine cancers. This led to an investigation to answer the questions “Is Zantac safe?” and “Is there a connection between Zantac and cancer?”.
While the small amounts initially found are not necessarily dangerous in and of themselves, the FDA also found that over time, the levels of NDMA can increase. At these higher levels, especially when taken for long periods of time when the user is continuously exposed to these larger quantities of NDMA, the risk of developing cancer becomes much higher. Due to this concern, they made the decision to issue a recall of Zantac and ranitidine drugs to protect consumers from this threat to their health.
To help you further understand the dangers of taking Zantac and other ranitidine drugs, let’s take a closer look at the two main factors involved; ranitidine and NDMA.
What is Ranitidine?
According to Harvard.edu, ranitidine is a drug classified as a histamine-2 blocker. It’s primary purpose is to reduce how much stomach acid is produced. As such, it’s used to treat conditions related to overproduction of stomach acid, including stomach ulcers, intestinal ulcers, and GERD. Ranitidine is the active ingredient in Zantac and generic versions of the drug.
What is NDMA?
N-Nitrosodimethylamine (NDMA) is an organic chemical that can occur when the elements nitrite (“N”) and dimethylamine (“DMA”) — both of which are found in ranitidine — are combined. NDMA is found in a variety of pharmaceuticals and is a carcinogen (a substance that promotes the formation of cancer).
While very low levels of NDMA are also found in some food and water we consume, it is generally not enough to increase our risk of cancer. However, that is not what the FDA’s findings have led them to believe in the case of NDMA in Zantac and other ranitidine drugs. This is because the levels of NDMA in ranitidine increase over time and during the digestion process.
As these drugs are stored for long durations—from the start of the manufacturing process to when they make it into the hands of consumers and are physically ingested—the risk of cancer is higher. This is especially true for ranitidine drugs that are stored at higher temperatures, which further increases NDMA levels in the drugs.
Types of Cancer Zantac Has Been Linked to
The connection between Zantac and cancer has been determined to exist due to the fact that NDMA is carcinogenic. According to the World Health Organization, NDMA consumption is positively associated with gastric and colorectal cancer.

However, as the recall and investigation are fairly recent and lawsuits are currently underway, there are a lot of unknown factors. Some lawsuits are claiming that Zantac and other ranitidine drugs have caused other types of cancer, not limited to bladder cancer, intestinal cancer, stomach cancer, and esophageal cancer.
Zantac Alternatives
Without Zantac to rely on for relief from GERD and other conditions, you may find yourself back in the uncomfortable position that led to you begin taking the medication in the first place. The thought of going back to the days where you had to deal with chest pains, difficulty swallowing, disrupted sleep, and more, can be concerning to say the least. So, how can you avoid that scenario, without your Zantac prescription?
You should focus on finding another treatment method following the Zantac recall. But, what is an alternative to Zantac? Fortunately, you have several options depending on your preference and the recommendation of your doctor. Current options for Zantac alternatives include:
- Medications
- Medical devices
- Lifestyle changes
We’ll cover each of these options in greater detail in the following sections, so you can make an informed decision about the next phase of your treatment.
Alternative Medications
You will need to find a Zantac alternative that will help relieve your symptoms, without putting yourself at risk of NDMA side effects. There are several alternative medications you may be able to use to help with GERD symptoms and heartburn, including:
- Proton pump inhibitors (Prevacid, Prilosec, etc.)
- Histamine-2 blockers (for example, Pepcid)
However, there are also non-medicinal solutions that can help you break your dependency on prescriptions for good.
Medical Devices
If you are looking for a Zantac alternative or find that your reflex meds are not working, a medical device designed to help with GERD and acid reflux may be your best option for finding relief from discomfort and trouble sleeping comfortably. The company on the forefront of developing an alternative to Zantac and other anti-reflux medications is Amenity Health, Inc. The company developed and markets MedCline Reflux Relief System, a Class I medical device that treats nighttime GERD and acid reflux.

This acid reflux pillow positions users on an incline and optimizes side-sleeping in order to treat nighttime reflux, which has been shown to be the most dangerous form of reflux. Numerous clinical trials performed by research institutions throughout the US have shown that MedCline is far more effective than bed wedges and that quality of life is also significantly improved using the product. Compared to previous studies measuring the effectiveness of more power medications for reflux, MedCline has demonstrated superior symptom relief and health-related quality of life.
Better yet, MedCline is available without a prescription and can be purchased at MedCline.com. You may also use your HSA or FSA funds to purchase MedCline by visiting HSAstore.com or FSAstore.com.
Lifestyle Changes
In addition to switching medications or using MedCline’s Reflux Relief System, there are a few lifestyle changes that come recommended by the International Foundation for Gastrointestinal Disorders (IFFGD) you can make to help manage your symptoms:
- Avoid caffeine and alcohol
- Make changes to your diet
- Eat earlier in the evening to leave time for proper digestion
- Focus on weight loss
- Avoid medications that can trigger acid reflux and drink plenty of water with medications
Making these changes to your lifestyle can help with acid reflux, minimize your risk of exposing yourself to the potential side effects of other prescription drugs, and prevent your from experiencing pill esophagitis.
Next Steps: What Should You Do If You’ve Taken Zantac in the Past?
First and foremost, if you are still taking your prescription to finish it out, schedule an appointment with your doctor immediately to come up with a plan to stop taking it and seek out one of the Zantac alternatives discussed above.

If you’ve previously taken Zantac or another ranitidine drug, you may be wondering whether you’ve been exposed to NDMA or even if you may have cancer. Schedule an appointment with your doctor as soon as possible in order to determine what steps are necessary, including potentially testing for cancer as well as helping you determine the best Zantac alternative for your circumstances.
If you find that you have developed stomach, liver, or bladder cancer due to taking Zantac, you may be eligible for compensation in the currently ongoing lawsuit against the manufacturer. It may be in your best interest to contact a lawyer to see if you qualify as part of the lawsuit. While monetary compensation is not the same as being cancer-free, it can help make treatment costs more manageable and provide you with some peace of mind.
Find Relief from GERD and Acid Reflux without Zantac
The Zantac recall and cancer scares have left many GERD and acid reflux sufferers in a state of uncertainty and dread. However, with the help of your doctor, you can find a safer alternative such as our Reflux Relief System, that will help you find the relief you need, and ultimately, enjoy a better quality of life.
Say goodbye to nighttime Acid Reflux & GERD pain without sacrificing comfort.Related Product
Reflux Relief System
$249.99 BUY NOW
References
Related Product
Reflux Relief System
$249.99
Say goodbye to nighttime Acid Reflux & GERD pain without sacrificing comfort.
Subscribe
Sign up to get the latest on sales, new releases and more …
Text SHOULDER to+1 (844) 942-0170to learn more and get discounts on our Shoulder Pain Relief System.
About MedCline
MedCline was founded in 2011 by Carl Melcher, M.D, who was a life-long sufferer of GERD. Dr. Melcher wanted to help the millions of GERD patients with a natural treatment alternative utilizing positional therapy. Since development, the Reflux Relief System has been validated in 7 clinical trials. Aiming to help other medical conditions with positional therapy, MedCline has also developed a Shoulder Relief System for those who suffer with chronic shoulder pain at night. Both MedCline Relief Systems are providing much-needed relief for those suffering from nocturnal acid reflux and/or nighttime shoulder pain to get quality, restorative sleep leading to a higher health-related quality of life.
To learn more about acid reflux relief, visit our Reflux Relief System Page.
To learn more about shoulder pain relief, visit our Shoulder Relief System Page.
